Breadcrumb
- Home
- Research
Who we are

#DiminutiveDosageForm

#ParticleCoating
#ASD-Downstream

#Co-Processing
Welcome to The Hiew Laboratory for Pharmaceutical Formulation and Processing Research!
Today, the development of oral dosage forms is increasingly plagued by challenges associated with poor solubility or permeability, or both. Apart from formulation-related challenges, the manufacturing of oral solid dosage forms also presents its own set of obstacles. Some of the unmet needs in oral drug delivery include drug delivery systems for specific populations such as the pediatric and geriatric populations, as well as drug delivery systems for targeted delivery of emerging therapeutics. Furthermore, improved excipients are needed to support the adoption of innovative manufacturing technologies. The unmet needs of the pharmaceutical industry can be addressed through advanced manufacturing approaches using innovative technologies. Our lab is interested in using particle engineering strategies to address contemporary delivery challenges, including those faced by emerging therapeutics. We are currently working on the following:

#DiminutiveDosageForm
Diminutive dosage forms such as pellets, mini-tablets, and granules are critical for oral drug delivery to specific populations such as the pediatric and geriatric populations. Using the state-of-the-art STYL'One Evo compaction simulator, we are interested to study the art and science of mini-tablet compression as well as the challenges associated with the formulation and development of this dosage form.

#ParticleCoating
Particle coating can be leveraged as a strategy to modify the surface and shape of individual particles. Particle coating can also be used to apply functional coatings to particles for taste masking, stability enhancement and/or release modification. With the FlexStream technology, we aim to use targeted surface deposition of particles as a strategy for improved drug delivery.
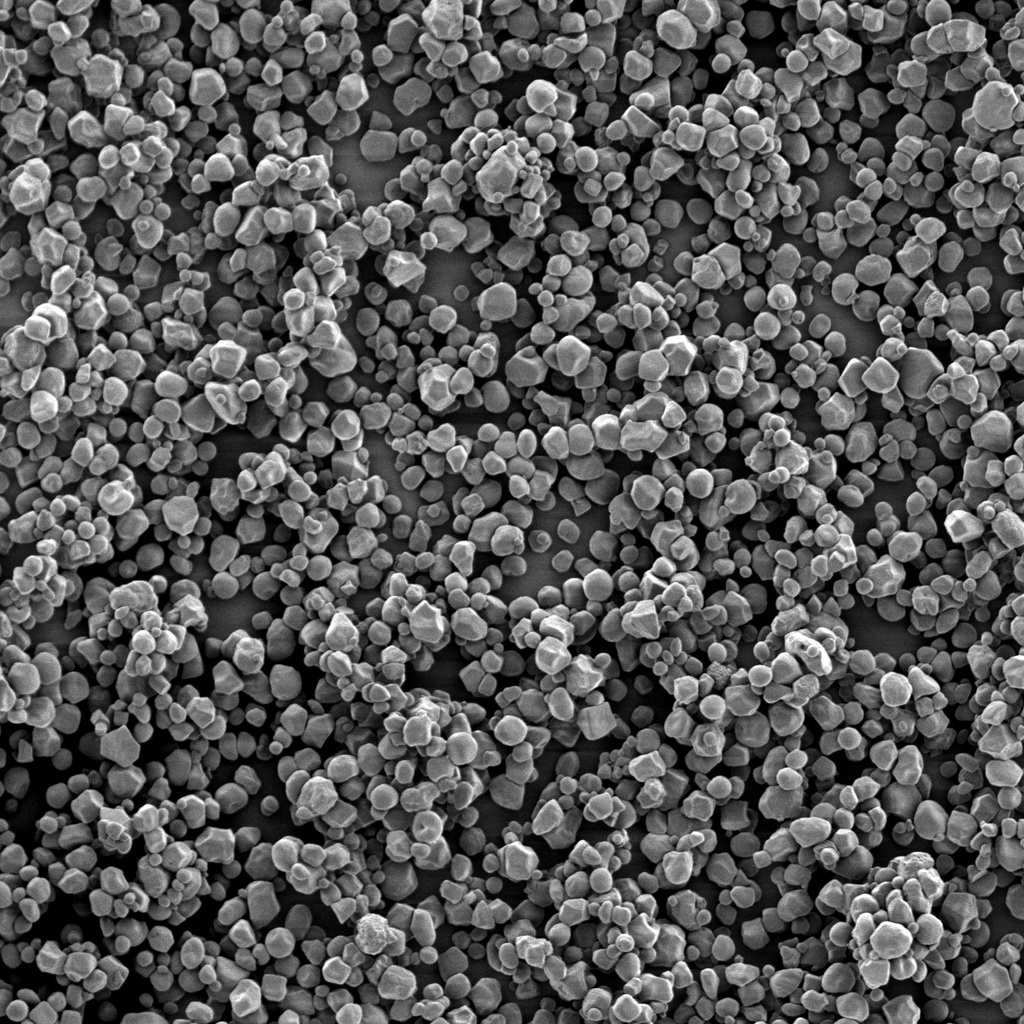
#Co-Processing
Today’s molecules cannot be formulated with yesterday’s excipients. As we move toward more advanced manufacturing approaches such as continuous manufacturing, functional excipients become increasingly important to accommodate the formulation needs of drug molecules. Rationale-driven excipients co-processing can be leveraged to achieve a specific target product profile. We plan to develop co-processed excipients with improved functionality for continuous manufacturing to support a more cost-effective and sustainable manufacturing process.
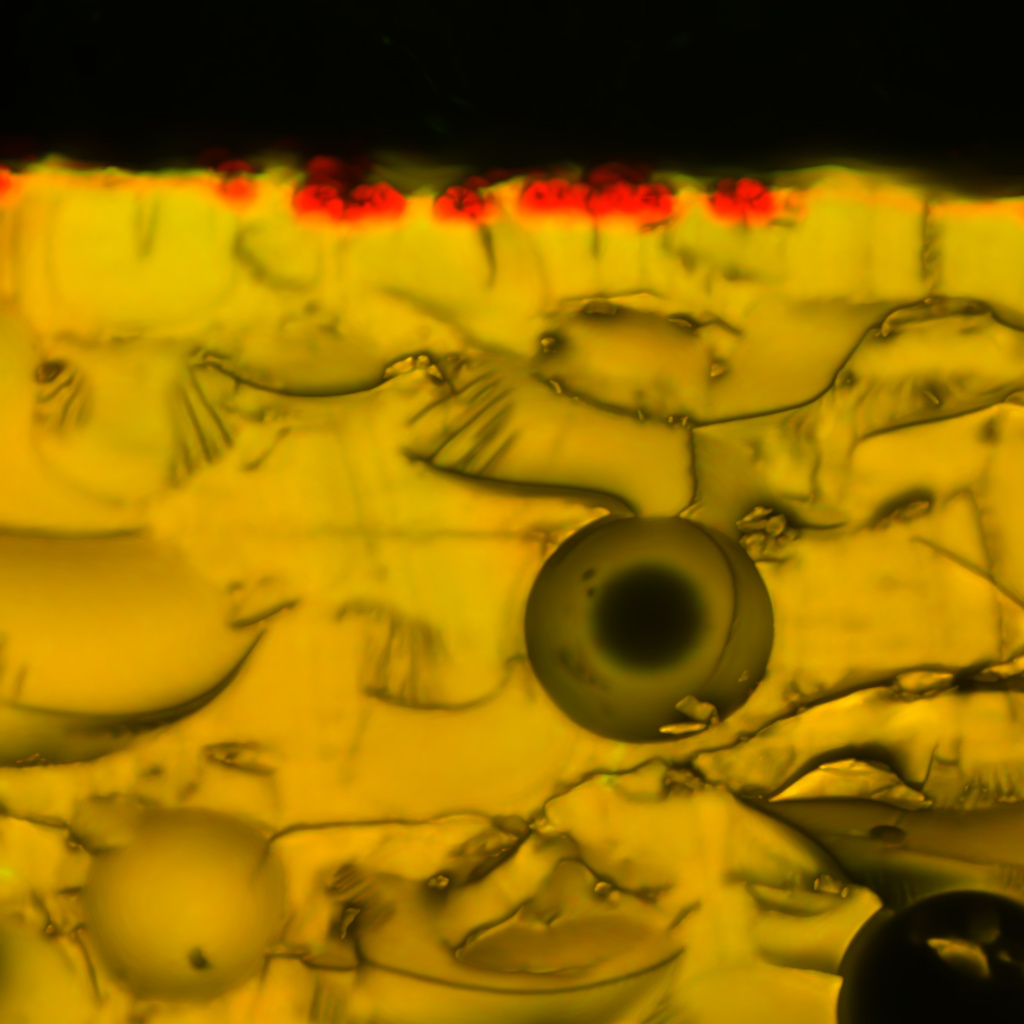
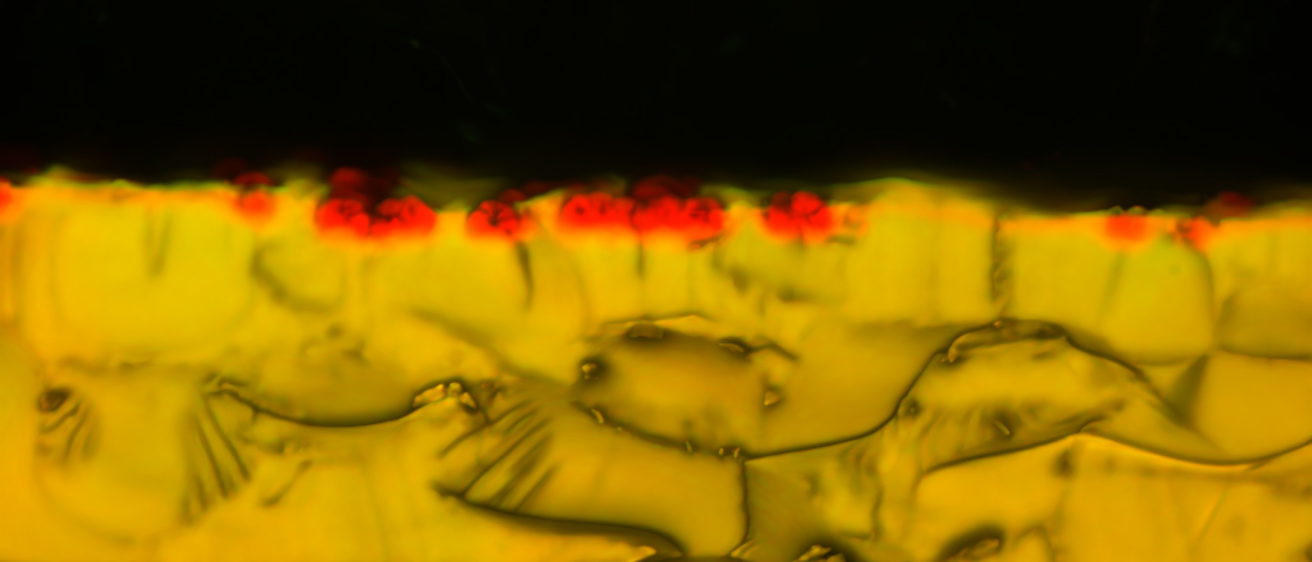
#DownstreamASDProcessing
Amorphous solid dispersion (ASD) is a popular enabling formulation strategy for poorly soluble drugs as drugs in the amorphous state can achieve supersaturation in the gastrointestinal tract, thereby conferring a bioavailability advantage compared to their crystalline counterparts. We are interested in exploring ASD downstream processing to further understand how excipient choices and downstream processing parameters affect drug release performance.